Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Multi Modal AI-Driven System for Enhanced Neurological Detection Using EEG, Retinal, and Brain Imaging Data
Authors: Prof. Swati Shilaskar , Shravani Raut , Satvik Vishnoi , Rajat Jambhulkar
DOI Link: https://doi.org/10.22214/ijraset.2024.65229
Certificate: View Certificate
Abstract
This study presents a novel approach to detecting neurological disorders by integrating electroencephalogram (EEG) signal analysis with retinal scans, brain imaging techniques (MRI and CT), and patient-reported data. The primary objective is to develop an AI-driven diagnostic tool that utilizes multi-modal data fusion and deep learning for feature extraction, significantly enhancing diagnostic accuracy and supporting personalized patient profiles. Advanced deep learning architectures are employed to identify meaningful patterns from each data source, while innovative fusion techniques synthesize these patterns to deliver comprehensive assessments. Key outcomes demonstrate substantial improvements in diagnostic precision, early detection capabilities, and real-time monitoring, facilitating timely clinical decision support. Validation through extensive testing on diverse datasets confirms a marked increase in diagnostic accuracy. The practical implications of this system include the early and accurate detection of neurodegenerative diseases, epilepsy, and psychiatric disorders, ultimately contributing to improved patient outcomes and tailored healthcare solutions.
Introduction
I. INTRODUCTION
The detection and diagnosis of neurological disorders, such as epilepsy, depression, and Alzheimer's disease, are crucial for timely intervention and effective treatment planning. Electroencephalogram (EEG) signals offer valuable insights into the brain's electrical activity, making them a promising tool for diagnosing these conditions. However, analyzing EEG signals presents significant challenges due to their complex and dynamic nature, often requiring specialized expertise and computational resources. Current state-of-the-art (SOTA) methods for EEG signal analysis face several limitations, including reliance on limited datasets, computational inefficiency, and difficulties in interpreting results accurately.
Existing studies have highlighted the need for more robust and efficient methods for EEG signal analysis to overcome these challenges and improve diagnostic accuracy. For instance, Alturki et al. (Year) proposed a unified framework for classifying various neurological disorders using machine learning techniques, demonstrating promising results in identifying diverse abnormalities from EEG signals [2]. Similarly, Al Fahoum and Zyout (Year) introduced a novel integrated methodology combining phase space reconstruction and deep neural networks for early detection of neurological abnormalities, addressing limitations associated with current SOTA methods [1].
To address the gaps in existing research and enhance the accuracy of neurological disorder diagnosis, this study proposes a novel approach that integrates advanced signal processing techniques with machine learning algorithms. Specifically, we aim to leverage discrete wavelet transform (DWT) and intelligent techniques to extract informative features from EEG signals and improve diagnostic accuracy. Our proposed system will offer several key features, including real-time analysis, hardware-software co-design, and low-power operation, making it suitable for deployment in resource-constrained environments such as wearable devices or remote healthcare systems. The significance of our proposed system lies in its potential to revolutionize the diagnosis of neurological disorders by providing accurate and timely insights into patients' brain health. By addressing the limitations of current methods and offering a more efficient and reliable solution, our system has the potential to improve patient outcomes and quality of life.
The structure of this paper will consist of an introduction to EEG signal analysis and neurological disorder diagnosis, a detailed description of our proposed methodology, experimental results and evaluation, discussion of findings, and conclusion. Through this comprehensive approach, we aim to contribute to the advancement of EEG-based diagnostic tools and pave the way for more effective management of neurological conditions.
II. LITERATURE REVIEW
Numerous studies have explored the use of electroencephalogram (EEG) signals for depression detection. However, current state-of-the-art (SOTA) methods have limitations that restrict their overall effectiveness. These limitations include a reliance on datasets with limited scope and accessibility, a focus on analyzing a single dataset, and the use of deep learning architectures that are computationally expensive. To address these shortcomings, this study proposes a novel integrated methodology that combines phase space reconstruction and deep neural networks. This approach utilizes publicly available EEG datasets and reconstructed phase space analysis to capture the complex EEG patterns associated with depression. Additionally, it incorporates a deep neural network component to ensure optimal efficiency and accurate classification. Overall, the proposed method offers a significant advancement in enhancing the accuracy of depression detection and provides the base for EEG-based depression assessment tools applicable to real-world settings.[1] Electroencephalogram (EEG) signal analysis is a well-established method for diagnosing neurological disorders such as epilepsy and autism spectrum disorder (ASD). This study investigates different feature extraction and classification techniques to improve the accuracy of diagnosing these conditions. The researchers developed a single system that can diagnose one or two neurological diseases simultaneously. They analyzed two different modes of EEG signals: single-channel and multi-channel. Independent components analysis (ICA) was used to remove artifacts from the EEG dataset. Then, the EEG dataset was segmented and filtered to remove noise and interference using an elliptic band-pass filter. Next, feature extraction was performed on the filtered signal using discrete wavelet transform (DWT) to decompose the signal into its sub-bands. Statistical methods were then used to extract features from the EEG sub-bands. These features were fed into four different classifiers to classify them according to their corresponding classes. The results showed that the combination of DWT with Shannon entropy (SE) and logarithmic band power (LBP) produced the highest accuracy. Overall, the classification accuracy reached 99.9% using SVM and 97% using ANN for the three-class single-channel and multi-channel modes, respectively. [2]
The diagnosis of brain disorders is a critical challenge in healthcare. Electroencephalogram (EEG) analysis is a widely used technique to diagnose various brain conditions. However, interpreting complex EEG signals can be challenging for medical professionals. This study proposes a novel method to improve the accuracy of brain disorder detection through innovative visual signal analysis. The researchers introduce a new time-frequency (TF) transform called the Forward-Backward Fourier Transform (FBFT) to convert EEG signals into images that can be easily interpreted by medical professionals and deep learning algorithms. They then utilize convolutional neural networks (CNNs) to extract features from the TF images and classify brain disorders. Additionally, they introduce the concept of “eye-naked classification,” which integrates human expertise with the CNN-based classification for improved accuracy. Their results demonstrate the effectiveness of the FBFT method, achieving high accuracy rates for various brain disorders, including epilepsy, Alzheimer’s disease, murmur, and mental stress. Furthermore, they incorporate a method to select the most informative EEG channels to further enhance classification accuracy. Overall, this study presents a promising new approach for visualizing EEG signals that can improve the diagnosis of brain disorders. [3]
While EEG is a valuable tool for diagnosing neurological diseases, analyzing the complex data is challenging and time-consuming. Existing methods often focus on single diseases, requiring multiple analyses. This study proposes a unified framework for classifying various neurological diseases using machine learning. The approach involves filtering raw EEG data, generating time-frequency spectrograms, extracting textural features, and employing machine learning classifiers. Tested on real-world datasets, this method shows promise for identifying diverse neurological abnormalities from EEG signals. [4]
The authors of this paper propose a method to automatically identify different neurological disorders from EEG signals. Their method extracts features from the EEG signals and uses a machine learning classifier to categorize them. The researchers investigated different feature extraction and classification techniques in order to improve the accuracy of diagnosing these conditions. They achieved high accuracy rates using a combination of discrete wavelet transform (DWT) and statistical methods for feature extraction, and a Support Vector Machine (SVM) classifier. [5] Early diagnosis of neurological disorders is crucial for timely intervention. EEG analysis is a promising tool, and machine learning offers exciting possibilities for early prediction. This study reviews cutting-edge AI methods for utilizing EEG data in detecting various conditions, including Parkinson's disease and schizophrenia. It compares different EEG signal analysis approaches and identifies challenges and future research directions.[6]
EEG is a valuable tool for diagnosing brain disorders, but existing machine learning methods often overlook feature redundancy and patient age. This study proposes a new framework to address these limitations. It extracts multi-scale features from EEG signals and incorporates patient age information. The framework achieves high accuracy using ensemble learning classifiers, making it a promising approach for EEG abnormality detection.[7] This study proposes a method for improving the accuracy of Parkinson's disease (PD) detection using EEG signals. The method combines Discrete Wavelet Transform (DWT) with different entropy measures and machine learning techniques.
The researchers found that four entropy measures, namely log energy entropy, threshold entropy, sure entropy, and modified Shannon entropy (TShEn), achieved high classification accuracy. They achieved an accuracy of up to 99.91% using a combination of DWT+TShEn and KNN classifier. The results suggest that this method has the potential to be a valuable tool for early detection of PD.[8]
This review examines the application of machine learning (ML) techniques to EEG signal analysis for diagnosing common neurological disorders. It focuses on epilepsy, Attention-deficit/hyperactivity disorder (ADHD), and Alzheimer's disease. The review explores EEG signal processing methods, discusses datasets used in research, and analyzes limitations and future directions for this promising diagnostic approach.[9] This study investigates improving Alzheimer's Disease (AD) detection accuracy in early stages using EEG signals. It explores combining Hjorth parameters with common features and evaluates different signal decomposition (filtering, DWT, EMD) and classification (SVM, KNN, RLDA) techniques to achieve the most accurate AD detection.[10]
This study compared Random Forest (RF) and Linear Discriminant Analysis (LDA) for EEG-based diagnosis of brain disorders. It analyzed EEG data from 20 individuals with and without epilepsy. Feature selection using mutual information was employed. RF achieved significantly higher accuracy (95.85%), recall (91.33%), precision (88.28%), and specificity (96.99%) compared to LDA (68.84%, 42.21%, 31.43%, 76.33%). These results suggest that RF is a more accurate classifier for diagnosing brain disorders using EEG signals.[11]
Retinal Imaging as diagnostic tool: The role of the retina as an accessible extension of the brain has been emphasized in recent research, allowing for the early detection of neurodegenerative diseases such as Alzheimer's, Parkinson's, and multiple sclerosis using non-invasive imaging methods like optical coherence tomography (OCT) [11]. The retina's unique capability to exhibit morphological changes associated with these conditions makes it a valuable component of neurological diagnostics, complementing EEG and brain imaging data. Integration of retinal scans into multi-modal systems improves diagnostic capabilities by providing early biomarkers that are often not accessible through traditional methods, enabling continuous and non-invasive monitoring of disease progression.
II. METHODOLOGY
A. System Overview
- The proposed integrated AI-driven system for neurological disorder detection combines EEG signal analysis with retinal scans, brain imaging data (MRI, CT), and patient-reported data. The system architecture comprises the following stages: data acquisition, pre-processing, feature extraction, multi-modal data fusion, classification, and real-time monitoring. Fig 1 illustrates the flow from data acquisition to real time monitoring.
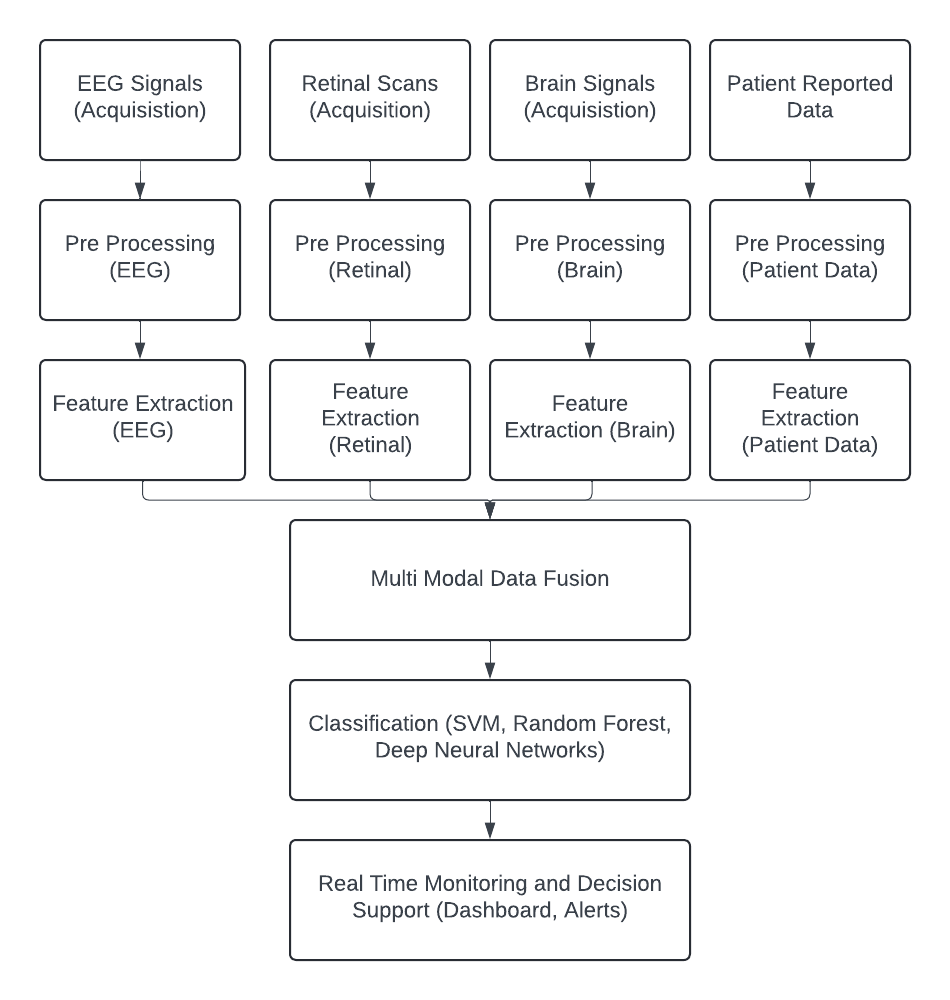
Fig 1: System Architecture Diagram Illustrating the Flow from Data Acquisition to Real time Monitoring
???????B. Data Acquisition
In the multi-modal data collection process, information is gathered from diverse sources, each contributing unique insights into neurological function and pathology. EEG signals are acquired using standard 10-20 electrode placement systems, allowing for the measurement of electrical activity in the brain with spatial specificity. Retinal scans, obtained through optical coherence tomography (OCT), provide detailed images of the retina, offering valuable information about ocular health and potential connections to neurological conditions. Brain imaging data, acquired via MRI and CT scans, offer comprehensive insights into brain structure and function, enabling the identification of abnormalities associated with neurological disorders. Additionally, patient-reported data, encompassing symptoms, medical history, and lifestyle factors, is collected through digital health records, providing subjective information that complements objective measurements from other modalities. This multi-modal approach to data collection ensures a holistic understanding of neurological health, incorporating both physiological and subjective perspectives for comprehensive assessment and diagnosis. Figure 2 visually represents the various sources from which multi-modal data is collected, illustrating the breadth and diversity of information utilized in the proposed AI-driven neurological disorder detection system.
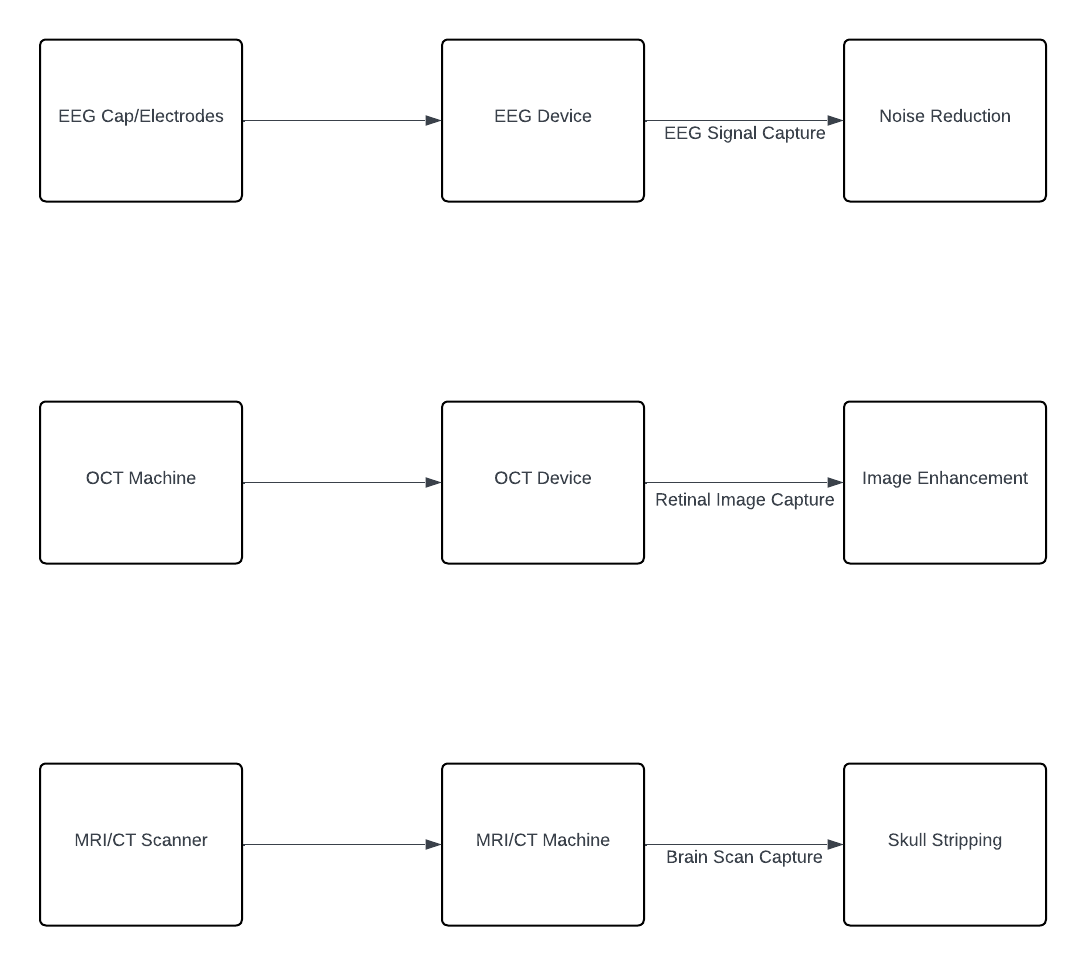
Figure 2: Sample Data Collection Setup for EEG, Retinal scans, and Brain Imaging
???????C. Data Preprocessing
In the data pre-processing stage, steps are taken to ensure the quality and consistency of the collected data across various modalities. For EEG signals, noise reduction is achieved through band-pass filtering techniques, followed by artifact removal using independent component analysis (ICA) methods. In the case of retinal scans, pre-processing involves enhancing the images through histogram equalization to improve contrast and noise reduction techniques for clearer visualization. Similarly, for brain imaging data, pre-processing steps include skull stripping to remove non-brain tissue, intensity normalization to standardize image intensities, and co-registration to align images from different modalities for accurate analysis. Additionally, patient-reported data undergoes standardization and normalization procedures to ensure consistency across textual and numerical information. These pre-processing workflows are essential to optimize the quality of the data before further analysis and integration into the AI-driven diagnostic system. Visual representations of these workflows for each data type would provide further clarity and understanding of the pre-processing methodologies employed. The Pre-Processing Workflow for each data type is illustrated in Figure 3.
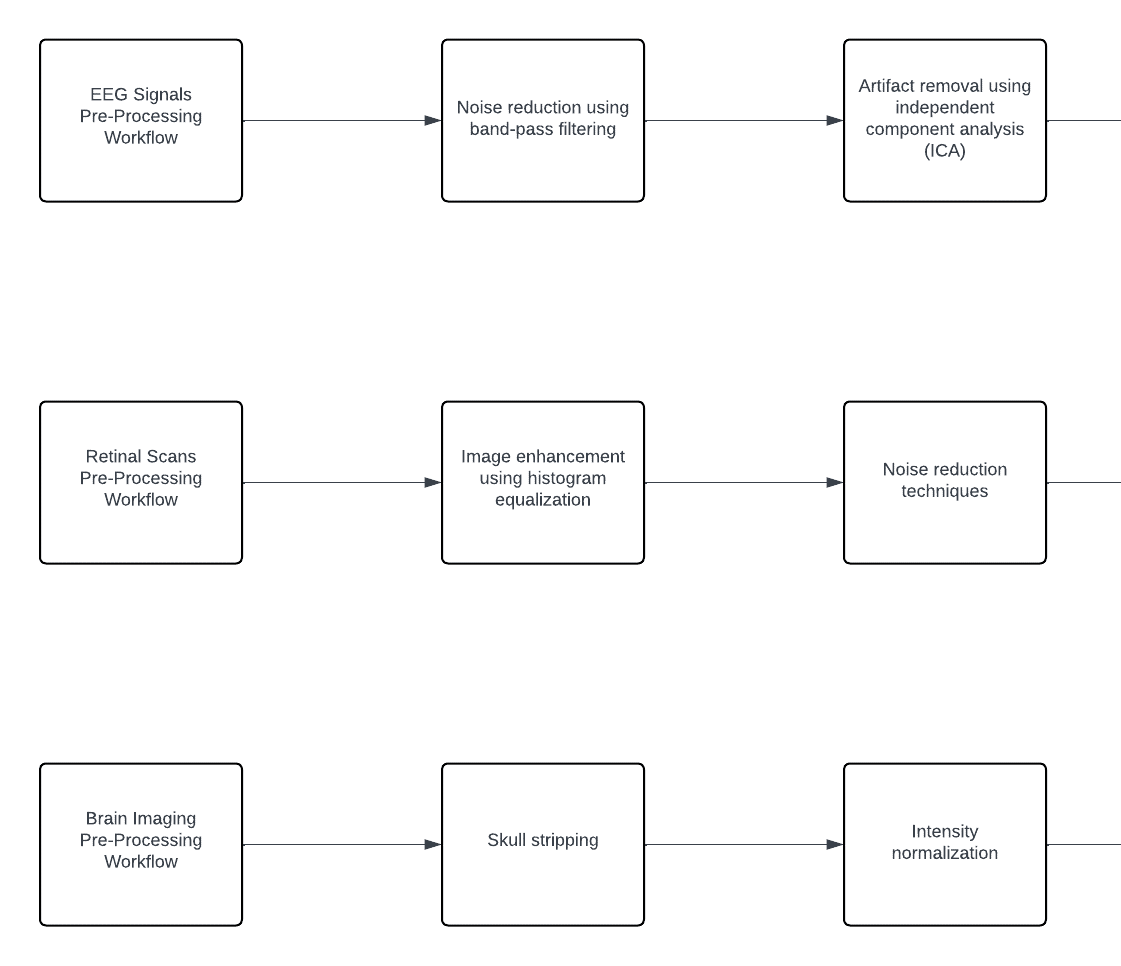
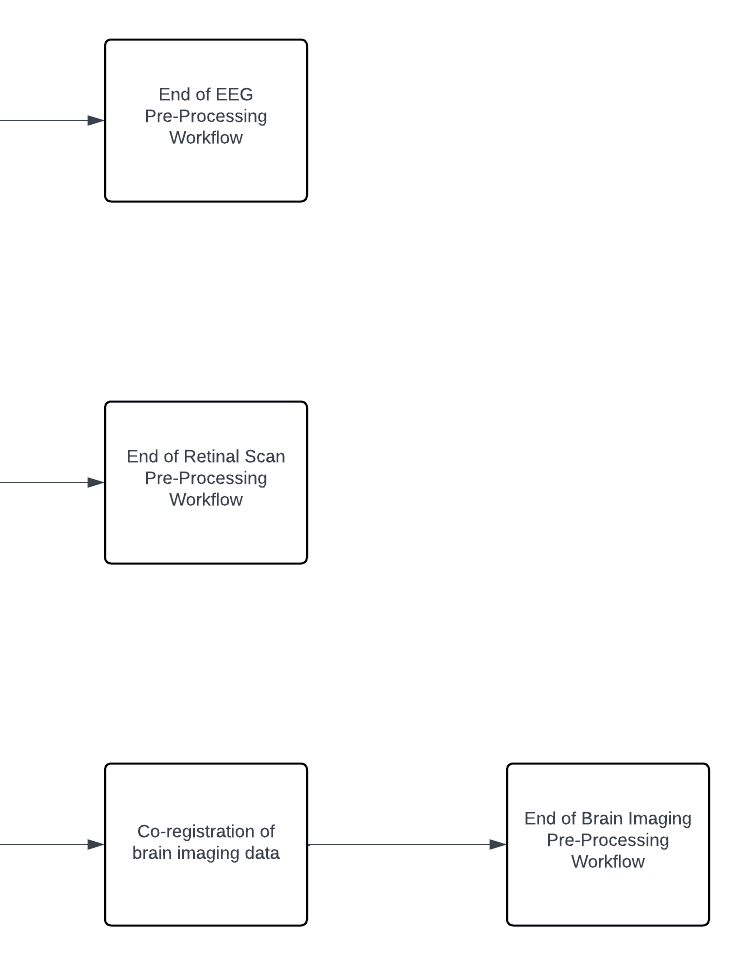
Figure 3: Pre-Processing Workflow for each data type
D. ???????Feature extraction
In the feature extraction stage, advanced deep learning architectures are employed to extract informative features from different types of data modalities. For EEG signals, temporal and spectral features are extracted using convolutional neural networks (CNNs) and recurrent neural networks (RNNs), allowing the system to capture both time-based dynamics and frequency characteristics inherent in EEG data. Similarly, for retinal scans, structural features are extracted using pre-trained CNNs such as VGG or ResNet, leveraging the powerful capabilities of these networks to discern intricate details and patterns within retinal images. In the case of brain imaging data, morphological and volumetric features are extracted using 3D-CNNs, enabling the system to analyze the complex three-dimensional structures present in brain scans with high accuracy and precision. Additionally, for patient-reported data, relevant features are extracted using natural language processing (NLP) techniques and machine learning models, allowing the system to derive insights from textual information provided by patients regarding their symptoms, medical history, and lifestyle factors. These deep learning models play a crucial role in extracting discriminative features from diverse data modalities, facilitating the subsequent stages of multi-modal data fusion and classification for accurate neurological disorder detection. Visual representations depicting the specific deep learning models utilized for feature extraction in each data modality would enhance understanding and illustrate the technical sophistication of the proposed system.
???????E. Multi Modal Data Fusion
In the multi-modal data fusion stage, features extracted from each modality are combined using sophisticated fusion techniques to enhance the overall diagnostic accuracy and reliability of the system. Feature-level fusion involves the concatenation of feature vectors extracted from different modalities, creating a unified representation of the data. This concatenated feature vector captures complementary information from each modality, providing a more comprehensive understanding of the underlying neurological conditions. To manage the high dimensionality of the combined feature space and alleviate the risk of overfitting, dimensionality reduction techniques such as principal component analysis (PCA) or t-distributed stochastic neighbor embedding (t-SNE) are employed. These techniques enable the extraction of the most informative features while preserving the essential characteristics of the data, facilitating efficient and effective analysis. Additionally, decision-level fusion techniques are employed to integrate predictions from individual models trained on each modality. Ensemble methods such as majority voting or weighted averaging are utilized to aggregate the predictions, leveraging the collective intelligence of multiple models to make more accurate and robust decisions. By combining predictions from diverse sources, decision-level fusion reduces the impact of individual model biases and uncertainties, leading to more reliable diagnostic outcomes. Moreover, ensemble methods provide a mechanism for capturing the inherent uncertainty in the data and making informed decisions based on consensus among multiple models. The data fusion process is crucial for leveraging the complementary strengths of different modalities and maximizing the information available for neurological disorder detection. By integrating features from EEG signals, retinal scans, brain imaging, and patient-reported data, the proposed system achieves a more holistic understanding of the underlying neurological conditions, enabling more accurate and personalized diagnosis and treatment recommendations. The visual information needed to illustrate the data fusion process would depict the sequential steps involved in feature-level fusion, dimensionality reduction, and decision-level fusion, highlighting the intricate interplay between different modalities and fusion techniques in enhancing diagnostic capabilities.
???????F. Classification
In the classification stage, integrated features extracted from multi-modal data are input into classification models to facilitate the diagnosis of neurological disorders with enhanced accuracy and precision. The process involves several key steps aimed at selecting appropriate models, training them effectively, and validating their performance robustly. Firstly, a variety of machine learning classifiers, including support vector machines (SVM), random forests, and deep neural networks, are evaluated to determine the most suitable candidates for the task at hand. Each classifier offers distinct advantages and may excel in different aspects of classification, such as handling non-linear data distributions or capturing complex patterns. Following model selection, the chosen classifiers undergo rigorous training and validation procedures using a labeled dataset. To ensure reliable performance assessment and mitigate the risk of overfitting, k-fold cross-validation is employed. This technique partitions the dataset into k subsets, with each subset used alternately as the validation set while the remaining subsets serve as the training data. By iteratively training and validating the models across multiple folds, the classification pipeline can effectively generalize to unseen data and produce robust diagnostic outcomes. The classification model pipeline encompasses a series of interconnected processes, from feature extraction and selection to model training and validation. Visualizing this pipeline can provide valuable insights into the intricate workflow involved in diagnosing neurological disorders. The visual representation should illustrate the sequential steps of feature integration, model selection, training, and validation, highlighting the interconnectedness and dependencies between each stage. By depicting the classification model pipeline comprehensively, researchers and practitioners can gain a deeper understanding of the methodologies employed and the considerations involved in developing a robust diagnostic system for neurological disorders.
???????G. Real Time Monitoring and Decision Support
In the real-time monitoring and decision support stage, the system continuously processes incoming data streams from various modalities to provide timely and actionable diagnostic support for clinicians. This capability is critical for early detection and intervention in neurological disorders, where prompt action can significantly impact patient outcomes. The real-time monitoring system is designed to handle and analyze data dynamically as it is received, ensuring that any emerging abnormalities are detected and addressed without delay.
One of the key components of this stage is the implementation of alert mechanisms. These mechanisms are programmed to trigger real-time alerts for clinicians whenever the system detects anomalies or patterns indicative of neurological disorders. Alerts are generated based on predefined thresholds and criteria derived from extensive data analysis and clinical guidelines. This proactive approach ensures that healthcare providers are promptly notified of potential issues, enabling them to take immediate action, whether it involves further diagnostic tests, modifications to treatment plans, or emergency interventions.
Complementing the alert mechanisms is a sophisticated dashboard interface. This visual interface is designed to provide clinicians with a comprehensive and intuitive view of patient data and diagnostic results. The dashboard displays individual patient profiles, summarizing key information such as medical history, ongoing symptoms, and current treatment plans. It also presents diagnostic results in a clear and accessible manner, highlighting any detected abnormalities and the system's confidence in its findings. Additionally, the interface tracks monitoring trends over time, allowing clinicians to observe the progression of a patient's condition and the effectiveness of ongoing treatments.
The integration of real-time monitoring and decision support features ensures that the system not only detects neurological disorders with high accuracy but also facilitates timely clinical decision-making. By continuously analyzing multi-modal data and providing real-time insights, the system enhances the responsiveness and effectiveness of healthcare delivery. This comprehensive monitoring and support framework is essential for managing chronic neurological conditions, where continuous observation and timely adjustments are crucial for optimal patient care.
Conclusion
The proposed system combines multi-modal data sources and advanced AI techniques to enhance the accuracy and reliability of neurological disorder detection. By integrating EEG, retinal, brain imaging, and patient-reported data, the system aims to provide comprehensive diagnostic support and improve patient outcomes.
References
[1] Al Fahoum, Amjed, and Ala’a Zyout. \"Early Detection of Neurological Abnormalities Using a Combined Phase Space Reconstruction and Deep Learning Approach.\" Biomedical Systems and Informatics Engineering Department, Hijjawi Faculty for Engineering Technology, Yarmouk University, Irbid, 21163, Jordan.J. Clerk Maxwell, A Treatise on Electricity and Magnetism, 3rd ed., vol. 2. Oxford: Clarendon, 1892, pp.68–73. [2] Alturki, Fahd A., Khalil AlSharabi, Akram M. Abdurraqeeb, and Majid Aljalal. \"EEG signal analysis for diagnosing neurological disorders using discrete wavelet transform and intelligent techniques.\" Sensors 20, no. 9 (2020): 2505.K. [3] Amer, Nisreen Said, and Samir Brahim Belhaouari. \"Exploring new horizons in neuroscience disease detection through innovative visual signal analysis.\" Scientific Reports 14, no. 1 (2024): 4217.Y [4] Tawhid, Md Nurul Ahad, Siuly Siuly, Kate Wang, and Hua Wang. \"Textural feature based intelligent approach for neurological abnormality detection from brain signal data.\" Plos one 17, no. 11 (2022): e0277555. [5] Sharma, Aarti, J. K. Rai, and R. P. Tewari. \"Identification of Various Neurological Disorders Using EEG Signals.\" In Advances in Computing and Data Sciences: Third International Conference, ICACDS 2019, Ghaziabad, India, April 12–13, 2019, Revised Selected Papers, Part I 3, pp. 95-103. Springer Singapore, 2019. [6] Jain, Abhishek, Rohit Raja, Sumit Srivastava, Prakash Chandra Sharma, and Jayesh Gangrade. \"Analysis of EEG signals and data acquisition methods: a review.\" Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization (2024): 1-26. [7] Wu, Tao, Xiangzeng Kong, Yunning Zhong, and Lifei Chen. \"Automatic detection of abnormal EEG signals using multiscale features with ensemble learning.\" Frontiers in Human Neuroscience 16 (2022): 943258. [8] Aljalal, Majid, Saeed A. Aldosari, Marta Molinas, Khalil AlSharabi, and Fahd A. Alturki. \"Detection of Parkinson’s disease from EEG signals using discrete wavelet transform, different entropy measures, and machine learning techniques.\" Scientific Reports 12, no. 1 (2022): 22547. [9] Safi, Mehrnoosh Sadat, and Seyed Mohammad Mehdi Safi. \"Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters.\" Biomedical Signal Processing and Control 65 (2021): 102338. [10] Zhang, Hao, Qing-Qi Zhou, He Chen, Xiao-Qing Hu, Wei-Guang Li, Yang Bai, Jun-Xia Han et al. \"The applied principles of EEG analysis methods in neuroscience and clinical neurology.\" Military Medical Research 10, no. 1 (2023): 67. [11] Yap, Timothy E., Shiama I. Balendra, Melanie T. Almonte, and M. Francesca Cordeiro. \"Retinal correlates of neurological disorders.\" Therapeutic advances in chronic disease 10 (2019): 2040622319882205.
Copyright
Copyright © 2024 Prof. Swati Shilaskar , Shravani Raut , Satvik Vishnoi , Rajat Jambhulkar . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET65229
Publish Date : 2024-11-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

